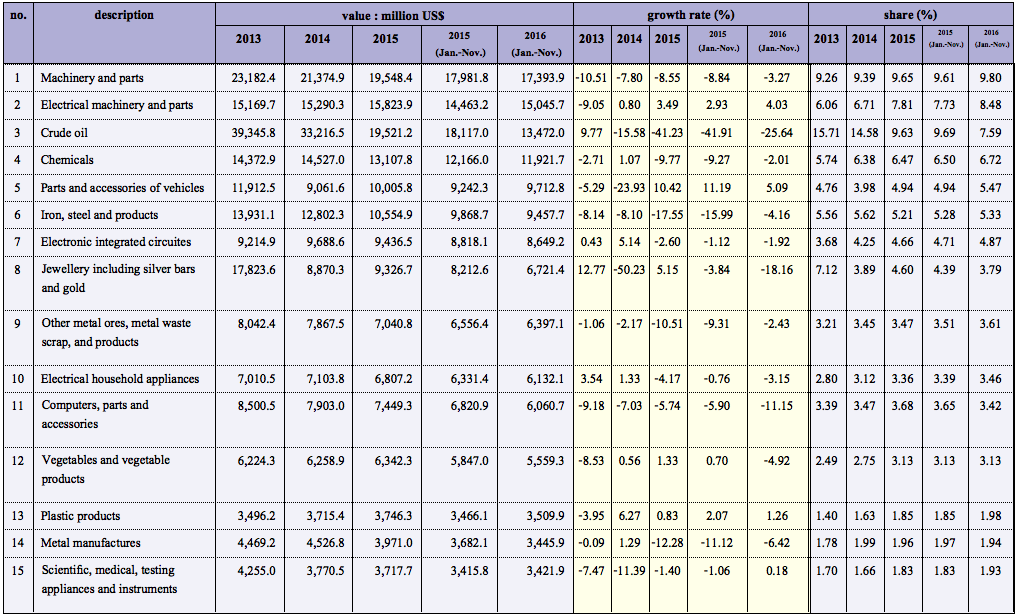
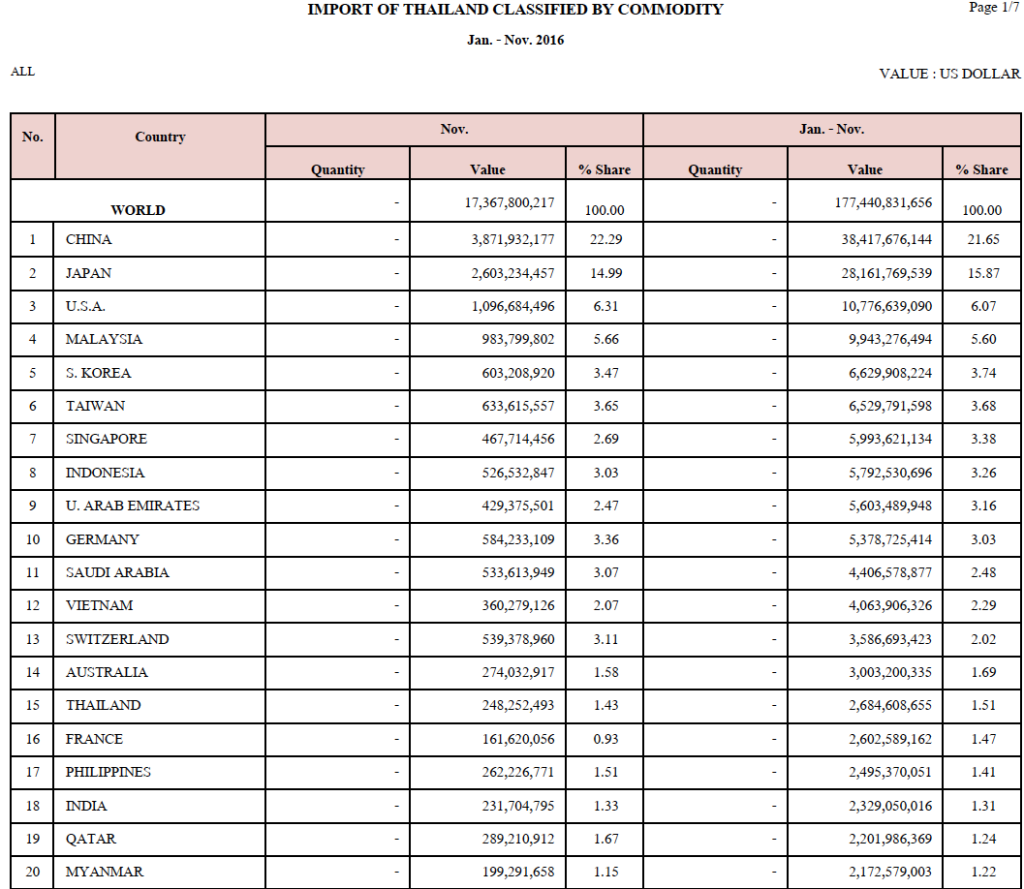
Due to the increased hygiene precautions from the COVID-19 pandemic, alcohol-based hand sanitizers and disposable medical face masks are facing severe shortages in Thailand. The Thai government has enacted several regulations to try to abate the shortage. The Ministry of Public Health (MOPH) has relaxed some regulations on the importation and production of these goods. In addition, the Department of Internal Trade of the Ministry of Commerce has acted to control the supply by setting up maximum allowable purchase price of domestic and imported face masks. Other regulations related to these products have been modified to counteract shortages—examples include reclassification of alcohol-based hand sanitizers, implementation of fast-track pathways for the domestic production of alcohol-based hand sanitizers, declaration of alcohol-based hand sanitizers and disposable medical masks as controlled goods, and so on. Regulations such as these will likely continue to change periodically, so healthcare entrepreneurs should check frequently that they are compliant with the latest updates.
Thailand’s Updated Regulations on Alcohol-Based Hand Sanitizers and Disposable Medical Masks
There have been three notable regulations pertaining to alcohol-based hand sanitizers. First, the MOPH has retracted its 2019 reclassification of alcohol-based hand sanitizers as medical devices (Medical Device Act B.E. 2251 (2008)), instead choosing to continue to categorize it as a cosmetics product (Cosmetics Act B.E. 2558 (2015)). The MOPH’s decision to cancel the reclassification serves to alleviate the shortage of alcohol-based hand sanitizers because the more stringent controls of medical devices would cause further delays in restocking. The second regulation enforces a minimum alcohol concentration of 65% w/w in alcohol-based hand sanitizers in order to gain registration as a controlled cosmetic. Finally, the Thai Food and Drug Administration (FDA) has also relaxed regulations to allow domestic pharmaceutical manufacturers, traditional drug manufacturers, and medical device manufacturers to produce and sell alcohol-based sanitizer without first obtaining a cosmetics manufacturing license.
The shortage of disposable face masks is much more acute than sanitizers. Hospitals have run out due to high public demand, low production scale, and consumer hoarding. People have taken advantage of the opportunities to gain additional income by making and selling masks as an alternative to commercial grade face masks. However, these homemade, reusable masks are not classified as medical devices and therefore, cannot be advertised as personal protective equipment against COVID-19. Only disposable medical masks, which are classified as low-risk medical devices, are regulated. Imported masks require product registration with the Thai FDA. Because of the pandemic, the Medical Device Control Division has agreed to facilitate all registration of disposable medical masks.
Price Controls
As both alcohol-based sanitizer and disposable medical masks are currently in high demand, the Thai Central Committee on Prices of Goods and Services (CCP) has published price controls for these goods. The manufacturer, importer, and distributor of alcohol-based hand sanitizer are required to give the CCP their pricing details and may not increase the price without CCP’s permission. For domestically manufactured disposable medical masks, the retail price must not exceed THB 2.50 (approx. USD 0.08) per mask. Currently, the price of imported masks has not been fixed, and the CCP only requires that the importer, distributor, and retailer must not mark up the price more than 10%, 10%, or 23%, respectively. Because only domestic masks are regulated with a fixed price (and because some traders do not obey the law in such a high demand market), the price of masks in the Thai market continues to rise.