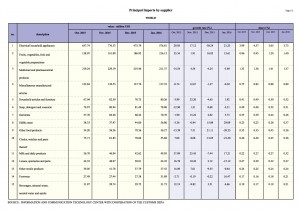
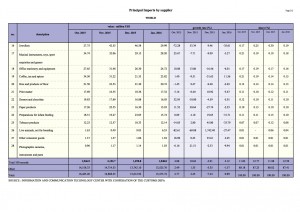
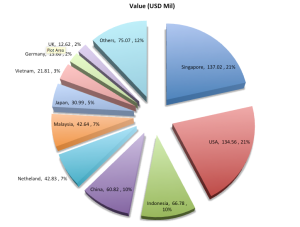
To better understand the food regulatory landscape in Asia, APFI has spoken to law firms that specialize in food law from five different countries across Asia, which include China, Thailand, Vietnam, Singapore and the Philippines.
In the second part of the series, we look at food safety regulations in Thailand. Alan Adcock, partner and deputy director, intellectual property and regulatory affairs, Tilleke & Gibbins, shares his advice.
How do food regulation and registration vary depending on the product or category?
The Thailand FDA classifies food products into four groups, depending on the risk level of the food, as follows:
- Specially controlled food:The foods in this group have a high risk level. This food group defines all quality standards, including labelling and production processes, and is tightly controlled. It includes foods for consumer risk groups such as infants.
The foods in this group are modified infant milk and modified infant milk formula, infant food and infant food formula, supplementary food for infants and young children, weight-control food, food additives, cyclamates, and steviol glycosides.
- Standardized food: This group comprises foods with a medium risk level. There are quality and labelling standards for each category of food, as in the first group. However, product owners are directly responsible for ensuring their products are in accordance with FDA regulations (i.e., regulations on formula, labelling, and safety and quality standards).
The foods in this group are coffee, edible salt, vitamin-fortified rice, alkaline-preserved egg, cream, electrolyte drinks, chocolate, tea, herbal tea, a few kinds of sauces (i.e., tomato sauce, chili sauce, papaya sauce, and flour sauce), ice, soy milk, drinking water, fish sauce, honey, peanut oil, butter oil, palm oil, coconut oil, fats and oils, mineral water, vinegar, butter, cheese, ghee, margarine and fat spreads, soy sauce, jam, jelly and marmalade, semi processed food, brine for cooking, royal jelly, royal jelly products, food supplements, beverages, cow’s milk, flavored milk, milk products, yoghurt, ice cream, and food in sealed containers.
Royal jelly products, food supplements, beverages, cow’s milk, flavored milk, milk products, yoghurt, ice cream, and food in sealed containers are tightly controlled.
- Food with labelling: This group comprises foods with a medium risk level. The FDA was formerly responsible for approving the labelling of food in this group. Currently, this group is no different from the second group. Product owners are directly responsible for ensuring their products are in accordance with FDA regulations (i.e., regulations on formula, labelling, safety, and quality standards).
The foods in this group are bread, husked-rice flour, sauces, meat products, flavoring, gelatine and jelly desserts, chewing gum and candy, ready-to-cook and ready-to-eat products, irradiated foods, GMO foods, and specially purposed food.
Specially purposed food (e.g., medical food, food for special persons, etc.) is tightly controlled.
- General food: This group comprises foods with a low risk level. Foods in this group do not require food product registration with the FDA.
The foods in this group are animals and animal products, plants and plant products, extracts or synthetic substances, nutrients, flour and flour products, premixed food for ready-to-cook products, seasoning, sugar, and spices.
In addition, the Thailand FDA provides basic levels of food safety that food manufacturing companies must adhere to in the form of ‘good manufacturing practices’ issued as notifications by the Ministry of Public Health. These are mandatory and discrete to certain food products. It is important for manufacturers to check regularly whether any are applicable to their products.
Have you seen a growth in the number of food and beverage companies entering the markets in Thailand?
We have seen growth not only in Thailand but also across the growing economies of Southeast Asia. Many clients encouraged us to expand our legal and regulatory services to other jurisdictions in the Association of Southeast Asian Nations, which we did in 2013 by opening our own fully independent law offices in Indonesia, Laos and Myanmar and subsequently in 2015 in Cambodia.
We find that more and more of our clients are looking for a ‘one-stop shop’ that can, for example, handle registration of their highly regulated products in multiple jurisdictions, which we provide with our cross-office Regulatory Affairs practice. We are also seeing more foreign clients pursuing patent litigation cases as local laws get tougher.
Food supplements and nutraceuticals are also becoming more prevalent in Southeast Asian markets. Interestingly, there is a growing number of local and foreign collaborative research and development projects focusing on indigenous flora for possible food and cosmetic applications.
We have seen a marked increase in the number of these projects across our offices here in Southeast Asia and we advise on the agreements, benefit-sharing assessments with local communities, plant variety and patent assessment and registration and, of course, manufacturing and distribution.
Which regulatory hurdles should manufacturers be aware of when entering the food market in Thailand?
Non-English language regulations require constant monitoring and vetting for importance to clients. Our regular involvement in local domestic industry associations also gives us opportunity to participate in regulatory drafting, consultation and bringing client perspectives to the attention of the regulators.
Regulatory non-compliance can result in fines and/or imprisonment depending on the violation and the type of product involved.
Will there be any major changes in Thailand’s food industry in the next few years?
The Thai legislative agenda can be difficult to predict, due to the changeable nature of Thai politics. However, the current government has been very enthusiastic about promoting the food industry as a driver of economic growth, and has made particular efforts to encourage startups in the industry.
In mid-2016, the government announced the establishment of a National Science Technology and Innovation Policy Office, which launched the Food Innopolis initiative. The initiative comprises a specifically designed science park, at which research and development companies can benefit from substantial tax incentives and various other forms of government support, many of which are guaranteed for between five and eight years.
This is indicative of a number of policies which the government has been seen to support, and it is reasonable to assume that the result will be a substantial increase in technologically innovative food and agricultural startups in the coming decade.
Source: https://www.tilleke.com/resources/apfi-food-safety-regulations-series-thailand